报告题目:Molecular mechanisms of chromosome segregation in meiosis
报告人:Yoshinori Watanabe 教授
主持人:何向伟 教授
时 间:2021年4月1日(周四)下午4点
地 点: 纳米楼457报告厅
报告人简介:
Education:
1980 April 1 -1984 March 31 Department of Biophysics and Biochemistry,
Faculty of Science, University of Tokyo
Awarded the degree of BSc in molecular biology
1984 April 1 -1989 March 31 Department of Biophysics and Biochemistry,
Faculty of Science, University of Tokyo
Obtained Ph.D.
Research and professional experience:
2004-2018 Professor at the Institute of Molecular and Cellular Biosciences,
University of Tokyo, Japan
2018-2020 Project research scientist, The Francis Crick institute, UK
2020 Professor at the Genome Damage Stability Centre, University of Sussex, UK
2020 Professor at the Science Centre for Future Foods, Jiangnan University, China
Research interest
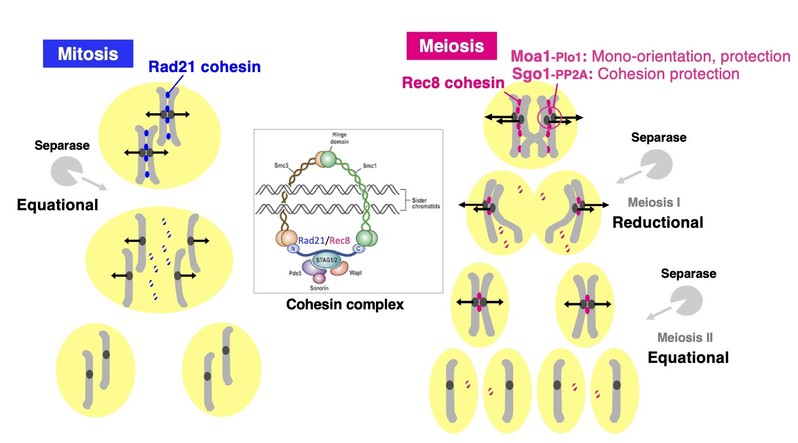
In mitosis, the establishment of sister chromatid cohesion is dependent on cohesin in S phase and maintained until metaphase when the sister chromatids are captured by spindle microtubules from opposite poles and aligned on the spindle equator. For the onset of anaphase, the anaphase-promoting complex (APC) triggers the degradation of securin, an inhibitory chaperone for separase that cleaves the cohesin subunit RAD21 and removes cohesin complex along the entire chromosome. This removal of cohesin triggers the separation of sister chromatids and their movement to opposite poles, a process called equational division. However, during meiosis, the meiotic cohesin REC8 mainly replaces RAD21 along the entire chromosomes; one round of DNA replication is followed by two rounds of nuclear division, which results in four haploid nuclei or gametes. In the first division of meiosis (meiosis I), homologous chromosomes connected by chiasmata are captured from the opposite poles, whereas sisters are captured from the same pole (mono-orientation of kinetochores). At the onset of anaphase I, REC8 cohesin is cleaved by separase along the arm regions, but protected at centromeres until metaphase II. Thus, mono-orientation and centromeric cohesion protection are two hallmarks of meiotic kinetochore function, which are widely conserved among eukaryotic organisms (see Figure). There is increasing evidence that cohesion protection is mediated by the centromeric protein shugoshin (SGO) and its partner protein phosphatase 2A (PP2A), which antagonizes REC8 phosphorylation, a prerequisite of cleavage. For last two decades, meiosis specific kinetochore proteins have been identified only in two yeasts (Saccharomyces cerevisiae Spo13 and Mam1(monopolin subunit), and Schizosaccharomyces pombe Moa1). However, in 2015, we identified a meiosis specific kinetochore protein Meikin in mouse and characterized the function, revealing that Meikin is the functional homolog of Moa1 and Spo13, which are required for cohesion protection and mono-orientation of meiotic centromere/kinetochore. Thus, accumulating evidence suggest that the key mechanisms required for meiotic chromosome segregation are conserved from yeasts to plants and animals. Especially, the finding that the fission yeast rec8 mutation causes shift the chromosome segregation manner from reductional to equational was applied to plants to develop apomixis technology.